Research
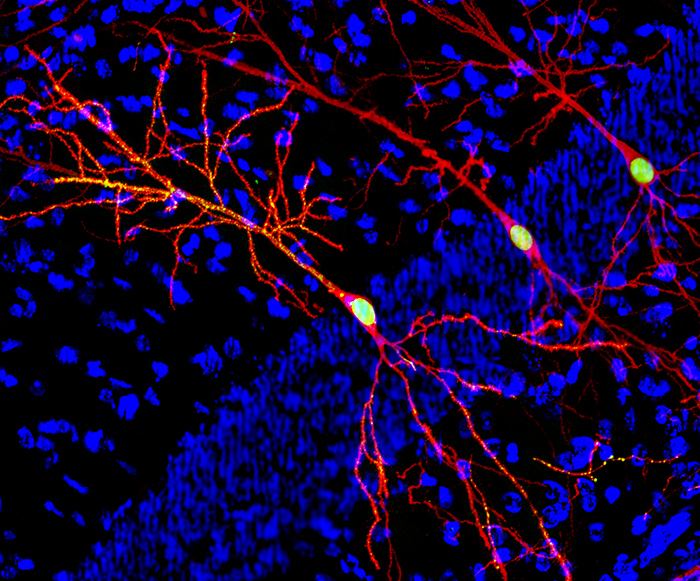
Cortico-Hippocampal Mechanisms of Context Memory
Episodic memories often persist throughout a lifetime, especially those associated with stressful experiences. Uncontrollable recall and rumination about past distressing memories, or an inability to extinguish maladaptive behavior rooted in such memories, lie at the heart of mental disorders such as anxiety, depression, and stress-related disorders. Extensive research established that the hippocampus plays critical roles in the formation of episodic memories. However, how the cortex and its interaction with the hippocampus contribute to the formation and retrieval of episodic memory is less understood. We research the contribution of the retrosplenial cortex (RSC), and its connections with the dorsal hippocampus (DH), to the encoding, consolidation, and retrieval of episodic-like contextual fear-provoking memories in mice. Our hypothesis is that the integration of DH inputs into RSC is essential for the processing of contextual memories throughout the earliest stages of memory encoding and the remote retrieval. In addition, rather than DH, the RSC acts as the gateway of information to other parts of the neocortex. We also investigate the role of three distinct DH-RSC projections in the formation, recent, and remote retrieval of context memories, and the contribution of layer 5 RSC neurons in long-term memory consolidation. Our approaches consist of several cutting-edge techniques. This includes conditional genetic and chemogenetic manipulations of individual RSC neuronal activities and DH-RSC projections. Furthermore, our lab performs optogenetic-electrophysiological approaches to dissect the functional neuroanatomy of DH-RSC projections and RSC layer-specific analyses of immediate early gene responses related to memory. Establishing the molecular and circuit mechanisms by which the RSC functions as a key contributor to memory will inform the development of novel treatment approaches for cognitive deficits in patients suffering from neurological and psychiatric disorders. Ideally, our findings will identify cortical pathways whose manipulation can bypass the requirement for the hippocampus in memory processing and thus potentially serve as a novel treatment option in patients with cognitive and affective disorders.
Mechanisms of Stress-Enhanced Aversive Conditioning
Traumatic stressful experiences can leave lasting painful memories in some individuals. In others they can cause dissociative amnesia, defined as an inability to consciously access memories of the traumatic events. Even so, these inaccessible memories can profoundly disrupt affective and social functioning of susceptible individuals. At a fundamental level, dissociative amnesia is thought to be rooted in state-dependent learning; meaning memories encoded in a certain affective or drug-induced state can be retrieved if the brain is again in the same state. Research into the neurobiology of state-dependent learning will give us a better understanding of the development of dissociative amnesia and stress-related psychopathologies. In our lab, we use mouse models to identify the molecular mechanisms of state-dependent fear conditioning and the circuit mechanisms by which they affect social behavior. As a key model of hippocampus-dependent episodic memory we use contextual fear conditioning. By using neurobiological approaches with mouse models, we propose to establish how such unconscious memories are formed and how they influence social behavior. A primary focus -based on our previous findings- are hippocampal extra synaptic GABAA receptors and how their sex-specific interactions with hilar oxytocin receptors contribute to state-dependent fear conditioning.
On the other hand, in the face of actual or anticipated threat, recalling negative memories offers important control of behavior. Nevertheless, in some cases this control can seep to non-threatening situations, a phenomenon known as overgeneralization of negative memories. Overgeneralization is a reliable cognitive phenotype of major depressive disorder, generalized anxiety disorder, and post-traumatic stress disorder. In our lab, we developed an animal model to study stress-induced generalization of negative memories (SIG) and determine its dependence on the episodic-like memory circuits. We found that SIG was strongly modified by social stress in both male and female mice. Furthermore, we used chemogenetic circuit manipulations during memory retrieval, to show that two sets of excitatory projections contribute to SIG: ventral tegmental area (VTA) to dorsal hippocampus (DH) and DH to the retrosplenial cortex (RSC). Based on the known roles of these projections, we suggest that (1) by targeting subcortical VTA circuits that provide valence signals to the DH, stress prioritizes the retrieval of negative over neutral memories, and (2) by forwarding such information to the RSC, stress engages cortical mechanisms that support the retrieval of general relative to specific memory features.
